SAP S4/HANA Certificates
"Quality certificates attest to the quality of a product for transparent and successful use."
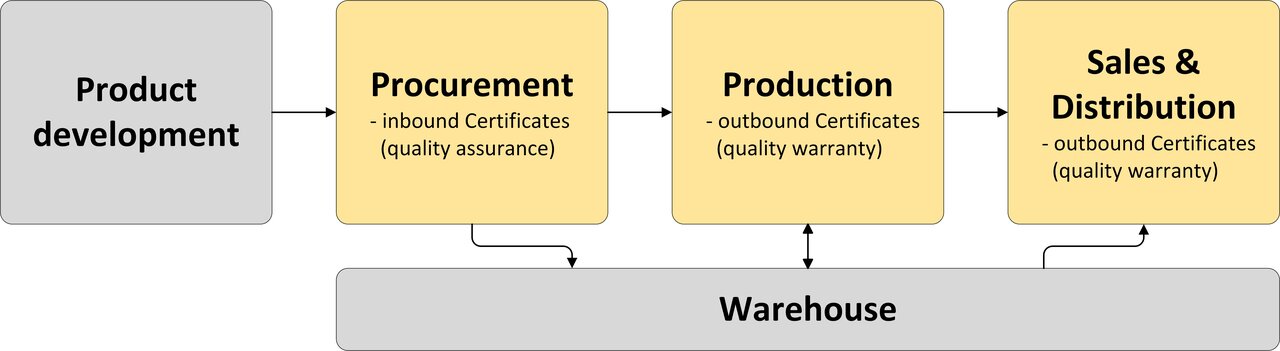
Generally, a quality certificate is stored or created automatically or semi-automatically for a delivery item. In individual cases, a certificate can also be requested ad hoc from an office with which there is no customer-supplier relationship. Therefore, if no delivery exists, a certificate can be created directly for an inspection lot or for a batch.
A quality certificate certifies the quality of a product. Chemical or physical properties of a product to be certified can be recorded as test results or documented as characteristics for a batch.
Customer-specific certificates can be created for clients who have special quality requirements that are based on customer-specific specifications (specification thresholds) or who require additional quality inspections. The content and layout can be adapted to customer requirements or based on legal guidelines.
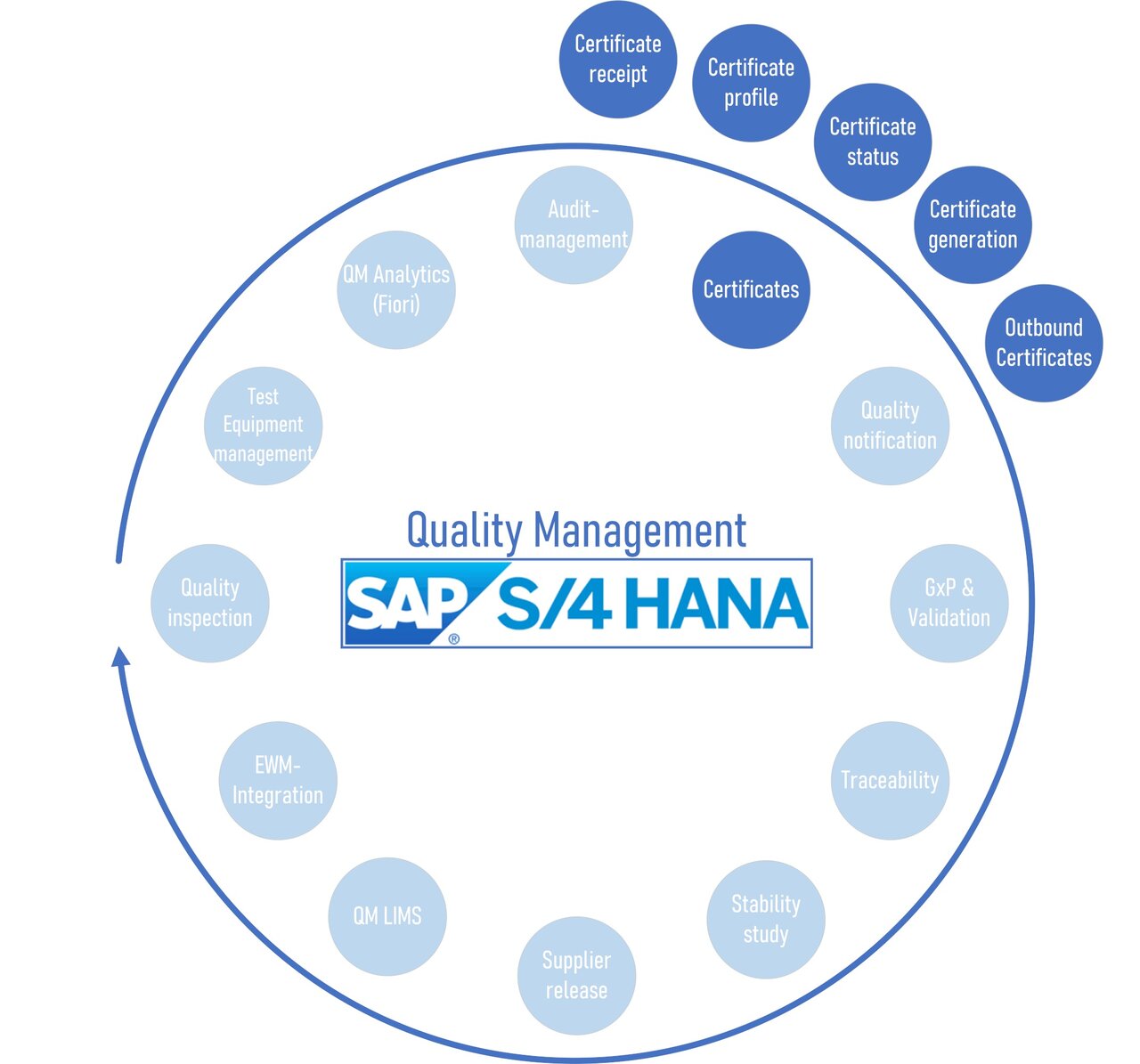
Illustration of the processes in the company
SI PRO Consulting Solutions for SAP QM with S4/HANA
SAP certificate receipt
If, in addition to compliance with technical delivery conditions and quality assurance agreements, you require a quality certificate for the goods in a purchase order from a particular vendor, you can monitor the receipt of the certificate - with or without inspection lot processing - independently of the time of the goods receipt. The certificate can be stored in the optical archive. If a certificate is outstanding, a reminder or reminder notice can be generated automatically.
SAP certificate profiles
A certificate profile controls the lot selection, the sequence of characteristics and the characteristic data. It contains header and characteristic data, although the last is not obligatory.
SAP certificate recipient
As a recipient of a certificate, you can provide for any person or address that has a role in the delivery item, such as the customer, a vendor, or the contact person.
SAP certificate creation
Generally, a certificate for a delivery item is created automatically at the time the material is delivered to a customer or at another time defined for the recipient. In addition, there are other ways to create a certificate, for example, directly for an inspection lot or for a batch.
SAP certificate status
The certificate profiles use the SAP status management. This documents the current processing status of the template. It depends on the status of the template whether certain functions may be executed or not. Each individual status thus fulfills two functions: (a) It informs you that a certain status has been reached (e.g. "Template has been released"). (b) It determines which activities may be executed next and which may not (e.g.: you can change the template; you cannot assign the template).
SAP certificate dispatch (EDI dispatch)
In the EDI scenario, you send the inspection results output on a quality certificate to your customer in formatted form. You can also send the quality certificate as a PDF file.
In principle, the quality certificate can refer to a delivery, an inspection lot, or a batch. The transfer of quality certificate data is therefore not necessarily linked to a purchase order or delivery. In fact, results of quality inspections that were carried out externally from the recipient's point of view can also be sent to the recipient's system and used flexibly there.
SAP QM Solutions

Chemical Industry
The Laboratory Information and Management System (LIMS) in SAP QM is based on processes and workflows commonly used in laboratories and thus offers numerous functions. Central components are the integrated sample management, test equipment monitoring and the possibility to create stability studies. The handling of the processes is supported by corresponding documents such as sample drawing instructions, inspection instructions, result reports and sample labelling.

Automotive Industry
The construction of complex machines, especially partially or fully automated ones, require a high degree of consistent dimensions and quality of the installed parts. The SAP quality certificate management system can be used to verify that these requirements are met by the suppliers. This ensures that changing requirements remain transparent and traceable at all times and thus guarantees continuous and complete documentation along the entire value chain.

GxP for Pharma
The manufacture of pharmaceuticals is subject to clearly defined legal requirements. SAP meets these compliance requirements with a range of functionalities that enable GxP-compliant implementation of QM processes, as well as supporting the necessary system validation. These functions guarantee continuous and seamless documentation along the entire product value chain. This enables complete change control through the integrated audit trail as well as batch tracking and tracing for all goods movements within the supply chain of integrated SAP logistics modules.
Customers
Why you should choose
SI PRO

SI PRO has been advising on SAP QM solutions since the earliest versions were released, and offers the most comprehensive level of best practice know-how.

Our consultants are familiar with specific issues, business processes, the latest SAP solutions, technologies, and appropriate procedures.

We can support you throughout your project’s entire life cycle, from analyzing your requirements to project planning, implementation, and maintenance.
Business Contact Managing Director
Lutz Dannemann
Tel. +4962130982615
E-Mail

Business Contact Partner
Daniel Mielach
Phone +498982939455
E-Mail








